|
|
| INTEGRATORI |
| NUTRIZIONE |
| BIOLOGICO |
|
|
| COMPOSIZIONE ALIMENTI |
| ALLENAMENTO |
| Oltre 80 consigli su alimentazione e allenamento con i pesi |
| PRODOTTI TIPICI D'ITALIA |
| RICETTE |
| Tante ricette dietetiche per Voi! |
|
|
ENGLISH
The amino acids are distinguished in ramificati (BCAA) and not ramificati: first they are three and they have the characteristic to tie to all the others, that is are essential for the construction of proteine.sono the Leucina, Isoleusina and alanina All the others instead are not essential, and arrange to you with first form various proteiche chains. The amino acids often are sold or in tablets or powder. For the persons who have difficulty in ingesting the pastiche council lively to assume integrators of amino acids in powder (also because many more convenient).
|
||||
|
Evolution of Dietary Antioxidants: Role of Iodine |
|||||
|
|
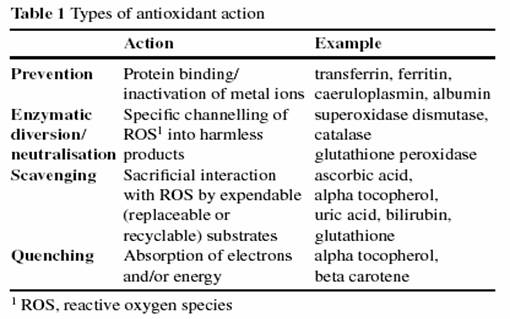
Lecture held at the “Thyroid Club” Annual Meeting of Bologna University, Feb. 6, 2007 By Sebastiano Venturi and Mattia Venturi Oxygen is a potent oxidant whose accumulation in terrestrial atmosphere resulted from the development of photosynthesis over three billion years ago, in blue-green algae (Cyanobacteria), which were the most primitive oxygenic photosynthetic organisms. In this review, we discuss the role of iodine in evolutionary strategies of antioxidant defense in plants and animals. A further aim of this paper is to provide a medical perspective. In fact, the importance of antioxidants as protective substances against many chronic and degenerative diseases, such as cancer and cardiovascular diseases has been studied for many years. But the utility of well-known antioxidant vitamins in some chronic diseases has not been recently supported by statistical data, and their benefits in cancer prevention have not been recently confirmed by epidemiological data (Bjelakovic et al. 2004; Hung et al. 2004; Lin et al. 2005; Sato et al. 2005; Tsubono et al. 2005; Morris and Carson 2003). In the wide range of antioxidants, we have recently hypothesized an “evolutionary hierarchy”, where the most ancient might be more essential than the “modern” ones in the developing stages of animal and human organisms (Venturi and Venturi 2004, 2006). |
||||
Deficiency of iodine, as a primitive antioxidant, seems to cause more damage in developing embryos than some other “modern” antioxidants. In fact, in pregnant women I-deficiency causes abortions and stillborns (Dunn and Delange 2001). Molecular iodine (I2) has the chemical capacity to non-specifically iodinate amino acids, proteins and lipids (Gottardi 1991). The oxidation of iodide by reactive oxygen species (ROS) like H2O2 has been studied since the early 1920s, and it is a necessary step to incorporate iodine into bioactive molecules (Bray and Caulkins 1921). These reactions yield a complex mixture of different iodine species. One factor that contributes to this complexity is the range of oxidation states associated with iodine species: -1 to +5, e.g. -1 (iodide – I-); +1 (hypoiodic acid - HOI); +5 (iodate - IO3) as shown in Table 1.
Table. 1. From Gottardi, 1991
Several oxidized iodine species formed from iodide oxidation have the potential to react with both water and I- (Bray and Caulkins, 1921). The iodine species that exist at a pH 7.4 are: iodide (I-), triiodide (I3 ), molecular iodine (I2), hypoiodious acid (HOI), hypoiodite ion (OI-) and the iodine anion (HI2O-). In 1985, Venturi suggested that the antioxidant biochemical mechanism of iodides was probably one of the most ancient mechanisms of defense from poisonous ROS as shown in Table 2 and in Table 3. 2 I- à I2 + 2 e- (electrons) = - 0.54 Volt ;
2 I- + Peroxidase + H2O2 + 2 Tyrosine à 2 Iodo-Tyrosine + H2O + 2 e- (antioxidants);
2 e- + H2O2 + 2 H+ (of intracellular water-solution) à 2 H2O Table. 2. Proposed antioxidant biochemical mechanism of iodides (From Venturi 1985)
2 I- + Peroxidase + H2O2 + Tyrosine, Histidine, Lipids, Carbons à à Iodo-Compounds + H2O + 2 e- (antioxidants)
Iodo-Compounds: Iodo-Tyrosine, Iodo-Histidine, Iodo-Lipids, Iodo-Carbons Table. 3. Proposed antioxidant biochemical mechanism of iodides, probably one of the most ancient mechanisms of defense from poisonous reactive oxygen species (Modified from Venturi 2003). Petersén et al. (1996), Küpper et al. (1998, 2002) and Gall et al. (2004) suggested that the production of volatile iodo-compounds by marine algae is a result of the development of photosynthesis, oxygen production and respiration some 3 billion years ago, and it is due to adaptation to light in order to reduce the amount of poisonous ROS, such as hydrogen peroxide, superoxide radicals and hydroxyl radicals.Increase of Oxygen in Earth’s Atmosphere and its Biological Consequences The evolution of oxygen-producing cells was probably the most significant event in the history of life after the beginning of life itself. Oxygen is a potent oxidant whose accumulation into the atmosphere forever changed the surface chemistry of Earth (Canfield 2005). Lane (2002), Wiedenheft et al. (2005) and Benzie et al. (2003) suggested that the evolution of oxygenic photosynthesis marks the dawn of oxidative stress and represents one of the greatest selective pressures imposed on primordial life. The association of molecular oxygen with abundant ferrous iron pools produced two major biological consequences. First, life dependent on the redox properties of Fe(II) had to contend with its oxidation and precipitation as Fe(III). Secondly, life had to contend with the toxicity of ROS generated by the partial reduction of dioxygen by ferrous iron. By the start of the Cambrian period 570 million years ago, or somewhat earlier, oxygen levels had apparently increased enough to permit rapid evolution of large oxygen-utilizing multicellular organisms. ROS potentially react with lipids, proteins, carbohydrates and DNA and thus interfere with the functions of cellular membranes, cell metabolism, cellular signaling, cell growth and differentiation. Oxidative stress seems to have been implicated as a causative process in the development of a vast number of degenerative diseases (Suzuki et al. 1997; Flohe et al. 1997; Yu 1994; Sies 1997). Stone (1988) studied the role of the primitive sea in the natural selection of iodides as a regulating factor in inflammation. This author reported that iodides have many non-endocrine biologic effects, including a role they play in the physiology of the inflammatory response. Iodides increase the movement of granulocytes into areas of inflammation and improve the phagocytosis of bacteria by granulocytes and the ability of granulocytes to kill bacteria. Early Developments in Antioxidant DefenseDuring evolution, endogenous protection systems have developed to counteract the deleterious effects of cellular oxidation. Protective antioxidant enzyme systems consist primarily of superoxide dismutase, glutathione peroxidase, catalase and peroxiredoxins. In addition to these endogenous systems, exogenous dietary antioxidants may help to prevent oxidative stress. In particular, mineral antioxidants present in the primitive sea, as some reduced compounds of Rubidium, Vanadium, Zinc, Iron, Cuprum, Molybdenum, Selenium and Iodine (I), which play an important role in electron transfer and in redox chemical reactions. Most of these substances act in the cells as essential trace-elements in redox and antioxidant metallo-enzymes. Some researchers hypothesized that the relative composition of many mineral trace-elements of the animal body is similar to the composition of the primitive sea, where the first forms of life began (Favier 1991). During evolution, in the last 500-400 million years (My) antioxidants of terrestrial origin developed in plants as many polyphenols, carotenoids, flavonoids, tocopherols and ascorbic acid. A few of these appeared more recently, in last 200-100 My, in fruits and flowers of angiosperm plants (Venturi 2004, 2006). In fact Angiosperms (the dominant type of plant today) and most of their antioxidant pigments evolved during the late Jurassic period. Plants employ antioxidants to defend their structures against ROS produced during photosynthesis. The human body is exposed to the very same oxidants, and it has also evolved an effective antioxidant system. Plant-based, antioxidant-rich foods traditionally formed the major part of the human diet, and plant-based dietary antioxidants are hypothesized to have an important role in maintaining human health. Benzie (2000, 2003) reported that the estimated daily intake of many selected antioxidants (such as antioxidant vitamins, polyphenols, carotenoids and flavonoids) decreased quantitatively from palaeolithic to modern
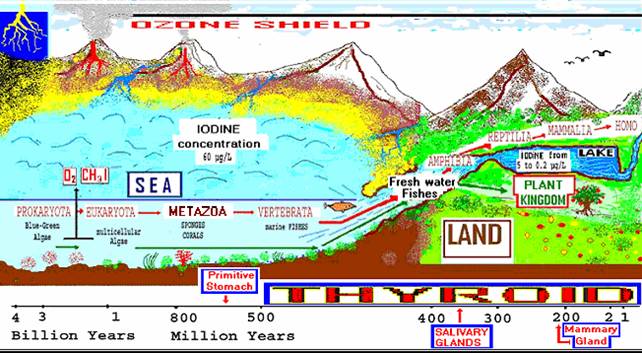
Iodide/iodine and Iodide/thyroxine: Evolutionary History of a Primitive AntioxidantOver three billion years ago, blue-green algae were the most primitive oxygenic photosynthetic organisms, ancestors of multicellular eukaryotic algae. Algae that contain the highest amount of iodine (1-2 % of dry weight) and peroxidase enzymes, were the first living cells to produce poisonous oxygen in the atmosphere (Obinger et al. 1997a, b; Venturi et al. 2000a, b). Therefore Venturi suggested that algal cells required a protective antioxidant action of their molecular components, in which iodides, through peroxidase enzymes, seem to have had this specific role (Venturi 1985; Venturi et al. 1987, 1993, 1999). In fact iodides are greatly present and available in the sea, where algal phytoplankton, the basis of marine food-chain, acts as a biological accumulator of iodides, selenium (and n-3 fatty acids) (Cocchi and Venturi 2000). The sea is rich in iodine, about 60 micrograms (mg) per liter, since this is where most of the iodine removed and washed away from the soil accumulated due to rains and the glacial ages (Elderfield and Truesdale 1980) (Fig. 1).
Figure 1. IODINE and EVOLUTION. Over three billion years ago, blue-green algae were the first living Prokaryota to produce oxygen and emit volatile halocarbons and CH3I in the atmosphere. For 700 million years, thyroxine has been present in fibrous exoskeletal scleroproteins of the lowest invertebrates. About 500-400 million years ago (Mya) some primitive marine fishes began to emerge from the iodine-rich sea and transferred to iodine-deficient terrestrial fresh waters. 400-300 Mya some vertebrates evolved in amphibians and reptiles and transferred to I-deficient land. Then, from primitive gastro-intestinal cells, a new “thyroidal” follicular organ developed, as a reservoir for iodine. In vertebrates, thyroid hormones became active in the metamorphosis and thermogenesis for a better adaptation to terrestrial environment. (From Venturi 2004).
The major iodine species in sea waters are iodate and iodide, along with smaller concentrations of molecular iodine, hypoiodous acid and iodinated organic compounds (Truesdale et al. 1995). Brown algae (seaweeds) accumulate iodine to more than 30,000 times the concentration of this element in seawater (Colin et al. 2003; Teas et al. 2004). Not much is known, however, on the iodine-concentrating mechanisms and on the biological functions of iodine in algae. Primitive marine prokaryotes seem to have an efficient active “iodide pump”, ancestor of the pump of multicellular eukaryotic algae and of mammalian iodide transporters. The mechanism of “iodide-pump” in the cells is very ancient and lacking of specificity, in fact, it is not able to distinguish iodide from other anions of similar atomic or molecular size, which may act as “pseudo-iodides”: thiocyanate, cyanate, nitrate, pertechnate, perchlorate (Wolff 1964). It is hypothesized that 80% of the Earth's oxygen is produced by planktonic algae, prochlorphytes, cyanobacteria and the free-floating unicellular microbes inhabiting the sea close to the surface. Up till now only one aspect of halogen metabolism, the production of volatile halocarbons, seems to have attracted more attention from researchers, because these compounds, and in particular the iodinated forms, have a significant impact on the chemistry of the atmosphere, and its ozone shield depletion (Carpenter et al. 1999, 2000). Halogen metabolism in marine algae involves enzymes known as haloperoxidases, which catalyse the oxidation of halides into hypohalous acids (Vilter et al. 1983; Vilter 1994, 1995; Gribble 1996; Pedersèn et al. 1996; Dembitsky et al. 2003; Gall et al. 2004). Since iodoperoxidase of Laminaria seaweeds is more efficient than the bromoperoxidase in the oxidation of iodide (Colin et al. 2003), this former activity may be more largely responsible for the uptake of iodide from seawater. There is an increased emission of iodinated halocarbons both from kelp beds at low tide during day-time (Carpenter et al. 1999, 2000), and from kelp plants incubated under high solar irradiance, caused by photo-oxidative stress, compared to plants kept in the shade (Gall et al. 2004). The green algae are hypothesized to have been ancestors of terrestrial plants. Recently Berking et al. (2005) confirmed Venturi’s hypothesis concerning antioxidant iodide and thyroxine in some marine invertebrates (polyps of the jellyfish Aurelia aurita) which contain iodide ions. Berking reported that in these invertebrates “the danger to be harmed by iodine is strongly decreased by endogenous tyrosine which reacts with iodine to form iodiferous tyrosine compounds including thyroxin. Both substances together, iodide and tyrosine, form an efficient oxidant defense system which shields the tissue against damage by ROS.” Spangenberg (1971) observed that when polyps of Aurelia are maintained for some weeks in iodide-free surroundings, it is possible to cause strobilation (metamorphosis) by applying iodine compounds including T4. (Polyps kept in normal seawater do not respond to strobilation). The interesting observation was that the polyps underwent strobilation when iodide was applied for 24 hours. But a 24-hour treatment with T4 was not sufficient. The treatment with T4 must carried out over a longer period of time to induce strobilation. It appears that T4 can deliver iodide, but this requires time. Recently Heyland and Moroz (2005) and Heyland et al (2006) reported that the oxidation of iodide to iodine in some marine invertebrates is a critical step for scavenging ROS, and that the reaction of iodine with tyrosine residues removes potentially poisonous iodine from the cell. In vertebrates, isolated cells of extrathyroidal iodide-concentrating tissues can produce protein-bound mono-iodo-tyrosine (MIT), di-iodo-tyrosine (DIT) and also some iodolipids (Banerjee et al. 1985; Aceves et al. 2005). This pathway for iodine organification involves iodine incorporation into specific lipid molecules. Iodolipids have been shown to be regulators of mammalian cellular metabolism. Iodine, reacting with double bonds of some polyunsaturated fatty acids of cellular membranes, makes them less reactive with ROS (Cocchi and Venturi 2000). Two iodinated lipids may be iodine autoregulation mediators: 6-iodo-5-hydroxy-8,11,14-eicosatrienoic acid (delta-iodo-lactone) and 2-iodohexadecanal. Delta-iodolactone has been found to be a potent inhibitor of proliferation of thyroidal and of some non-thyroidal cells (Banerjee et al.1985; Pisarev et al. 1988; Dugrilllon 1996; Venturi et al. 2000a, b; Cocchi and Venturi 2000; Cann et al. 2000; Aceves et al. 2005). According to Aceves et al. (2005) the percentage of iodine in cellular homogenate of breast tissue is about 40 % in lipid fraction and 50 % in protein fraction. Aceves also reported that in mammary gland homogenates from virgin rats, the addition of iodine in their diet significantly decreases lipid peroxidation. The family of peroxidase enzymes includes mammal, microorganism, plant, algal, and fungal peroxidases. Some of these peroxidases, known as haloperoxidases, use halide ions (iodide, bromide, and chloride) as natural electron donors, and have an antioxidant function in Cyanobacteria (Obinger et al. 1997, 1999; Venturi and Venturi 1999). Taurog (1999) reported that the relation between animal and non-animal peroxidases probably represents an example of convergent evolution to a common enzymatic mechanism. Heyland and Moroz (2005) suggest that exogenous sources of thyroid hormones (THs) (from food) may have been ancestral, while the ability to synthesize TH endogenously may have evolved independently in a variety of metazoans, resulting in a diversity of signaling pathways and, possibly, morphological structures involved in TH-signaling. In fact, increasing evidence suggests that THs also function in a variety of invertebrate species. The evidence of TH effects in invertebrates has been reviewed in Eales (1997) and Heyland et al. (2005).
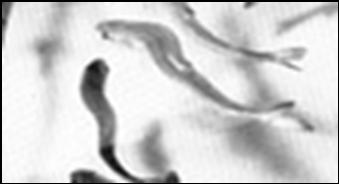
Evolution of Iodine from Non-hormonal to Hormonal Functions Since approximately 700-800 Mya thyroxine (T4) has been also present in fibrous exoskeletal scleroproteins of the lowest marine invertebrates (sponges, corals) (Roche 1952; Roche and Yagi 1952). Recent studies reported that THs are also present in unicellular planktonic alga (Dunaliella tertiolecta) and in echinoid larvae (sea-urchin) (Chino et al. 1994; Heyland 2004). These original sources of animal hormones might have been plants/algae in many cases, and could well have been independently derived from plants/algae in distinct lineages. The ancestral function of THs could also have been as feeding deterrents in algae and/or plants and the signaling functions in animals (Heyland and Moroz 2005; Eales 1997) might, therefore, have been acquired secondarily, perhaps even through horizontal transfer from their hosts or other co-associated microbes with more ancient relationships with the host. In waters the iodine concentration decreases step by step from sea-water to estuary (about 5 mg / L) and source of rivers (less than 0.2 mg / L in some Triassic mountain regions of northern Italy), and in parallel, salt-water fishes (herring) contain about 500-800 mg of iodine per kg compared to fresh-water trout about 20 mg per kg (Venturi and Venturi 1999; Venturi et al. 2000a, b, 2003). So, in terrestrial I-deficient fresh waters some trout and other salmonids (anadromous migratory fishes) may suffer thyroid hypertrophy or related metabolic disorders (Venturi et al. 2000a, b), as do some sharks in captivity. Youson and Sower (2001) reported that iodide-concentrating ability of the endostyle of sea lamprey was a critical factor in the evolution of metamorphosis and that the endostyle was replaced by a follicular thyroid, since post-metamorphic animals needed to store iodine following their invasion of freshwater. According to Manzon and Youson (1997) in some anadromous migratory fishes (sea lamprey and salmonids), iodine and TH play a role in initiation of metamorphosis, which is induced by the decline in serum of TH. After metamorphosis, when these adult marine fishes die in fresh-water after reproducing, they release their iodides and selenium, and n-3 fatty acids (Venturi et al. 2000a, b), in the environment, where they have a favorable role in food for life and health of native animals, bringing back upstream from the sea to I-deficient areas these essential trace-elements (Venturi et al. 2000a, b). In some I-deficient fresh-waters some salmonids may also suffers of “scurvy” and spinal curvature, due to dietary vitamin C deficiency.
Fig. 2 . Cultured salmons in freshwater showing nutritionally induced spinal curvature (scoliosis and lordosis) by vitamin C deficiency.
If these fishes are housed in I-rich sea-water then this disease improve, presumably because of the availability of other antioxidants in marine environment (Hardie et al. 1991). For this reason, we suggested that the antioxidant action of ascorbic acid developed firstly in the plants when, about 500-400 Mya plants began to adapting themselves to mineral (and iodine) deficient estuaries of rivers and land. Some biologists suggested that many vertebrates had developed their metabolic adaptive strategies in estuary environment (Purves et al. 1998). The role of iodine in marine and fresh-water fishes has not been completely understood, but it has been reported that I-deficient fresh-water fishes suffer of higher incidence of infective, parasitic, neoplastic and atherosclerotic diseases than marine fishes. Farrell et al. (1992) reported the absence of coronary arterial lesions in some elasmobranch fishes, living in I-rich sea-water. In October 7, 1999, the U.S.A. Committee of the House and Senate regarding "Marine Research" reported that " The Committee notes the unusually low incidence of cancer in marine sharks, skates, and rays and encourages basic research through the study of the immune system of these marine animals and the examination of bioactive molecules from shark, skate, and ray cells and tissues that have the potential to inhibit disease processes in humans." Yun et al.(2005) reported that marine vegetation concentrates iodine for its antimicrobial and antioxidant properties. In the amphibians, environmental iodine is the essential metamorphosis-factor and has an important role in the spectacular apoptosis on the cells of larval gills, tail, fins and gastro-intestine, and in adapting and transforming of aquatic animal (tadpole) to a “more developed” adult terrestrial animal (frog). In fact, programmed cell death, with nuclear changes and removal by phagocytic macrophages, occurs in a variety of organs, during amphibian metamorphosis, that is under the control of iodinated TH. Indeed, because of the massive cell death that occurs during a short period, amphibian organs serve as an ideal model system for the study of mechanisms underlying programmed cell death (Ishizuya-Oka et al. 2003; Ikuzawa et al. 2005).
Fig. 3. In amphibian metamorphosis iodides and thyroxine have an important role in the spectacular apoptotic action on the cells of gills, tail and fins, and in adapting and transforming an aquatic animal (tadpole) into a “more developed” terrestrial animal (frog).
TH induces apoptosis of larval cells and differentiation of pepsinogen-producing cells in the stomach of adult form of frog Xenopus laevis (Ishizuya-Oka et al. 2003). Upadhyay et al. (2002) reported that excess iodine has been observed to induce also apoptosis in thyrocytes and mammary cells. According to Upadhyay, mitochondria are important in iodination of different proteins; mitochondria are the central executioner of apoptosis and therefore may play an important role in carcinogenesis. Mitochondrial proteins from breast tissue are iodinated. Organification of iodine to proteins requires oxidative enzymes, H2O2 generating system and proteins in the vicinity, conditions favorable for the iodination of proteins, which exist in mitochondria under normal circumstances. Upadhyay observed that mitochondria isolated from the tumor (TT) and extra-tumoral tissue (ET) of human breast display significant uptake of iodine. Mitochondrial proteins were observed to be predominantly iodinated in ET but not in TT mitochondria. Treatment with iodine showed an increase in mitochondrial permeability transition of TT and decrease in ET. Iodine induced released factors other than cytochrome c from tumor mitochondria, which initiated apoptosis in vitro. Iodine in Terrestrial Organisms When about 500-300 Mya some living plants and animals began to transfer from the sea to rivers and land, environmental I-deficiency was a challenge to the evolution of terrestrial life (Venturi 2000a). In fishes, plants and animals the terrestrial diet became deficient in many primitive marine trace-elements, including iodine and selenium. Terrestrial plants slowly optimized the production of “new” endogenous antioxidants such as ascorbic acid, polyphenols, carotenoids, flavonoids, tocopherols, some of which became essential “vitamins” in the diet of terrestrial animals (vitamins C, A, E). According to Coic and Coppenet (1990) and Lamand (1991), iodine and selenium became no longer necessary to many plants. In a different way, some chordates, about 500 Mya, began to use also the “new” thyroidal follicles, as reservoir for iodine, and to use the thyroxine in order to transport antioxidant iodide and triiodothyronine into the peripherical cells. Triiodothyronine (T3), the biologically active form of thyroid hormone in vertebrates, became active in the metamorphosis and thermogenesis for a better adaptation to terrestrial fresh-waters, atmosphere, gravity, temperature and diet (Fig. 1). Extrathyroidal or peripheral TH metabolism is mediated by deiodinases [type 1 deiodinase (D1), type 2 deiodinase (D2) and type 3 deiodinase (D3)]. Deiodinases deliver from iodothyronines into peripheral cells atoms of iodide without hormonal action. D3 protein is also expressed by granulocytes and monocarboxylate transporter 8 (MCT8), a novel very active and specific thyroid hormone transporter is also present at the site of inflammation. Thyroxine, reverse-T3 and iodothyronines seem to be important as antioxidants and inhibitors of lipid peroxidation (Oziol et al. 2001; Berking et al. 2005), and more effective than vitamin E, glutathione and ascorbic acid (Tseng and Latham 1984). Moreover the new terrestrial diet harbored plant iodide-transport inhibitors such as thiocyanates, cyanates, nitrates and some glycosides (Wolff 1964; Brown-Grant 1961). Many plant substances that inhibit iodide-transport seem to have antiparasitic activity (Wolff 1964). In previous work, Venturi et al.(2000a) reported that, contrary to amphibian metamorphosis, in the mammals the thyroidectomy and hypothyroidism might be considered like a sort of phylogenetical and metabolical regression to a former stage of reptilian life. In fact, many disorders, similar to reptilian features, such as a dry, hairless, scaly, cold skin and a general slowdown of metabolism, digestion, heart rate, nervous reflexes with lethargic cerebration, hyperuricemia, and hypothermia seem to afflict hypothyroid humans. The new hormonal action was made possible by the formation of T3-receptors (TH-Rs) in the cells of vertebrates. First, about 500 Mya, in marine chordates, the primitive TH-Rs with a metamorphosing action appeared and then, about 250-350 Mya, in the birds and mammalians, others more recent TH-Rs with metabolic and thermogenetic actions were formed. TH-R genes are indeed c-erbA oncogenes, which have been implicated as tumor suppressor genes of non-thyroidal cancers and are altered in some human gastric and mammary cancer (Wang et al. 2002; Li et al. 2002). Role of Iodide in Animal Cells In 2001, Hays reported in “Thyroid ” journal that “It is surprising that the precise total iodine content of the human body remains uncertain after many years of interest in iodine metabolism … and that extra-thyroidal iodine pool remains a matter of speculation and also the chemical nature non-thyroidal iodine is unknown.” In humans, the total amount of iodine is about 25-50 mg and about 50-70 % of total iodine is non-hormonal and it is concentrated, via NIS, in extrathyroidal tissues (Venturi 2000a, b).
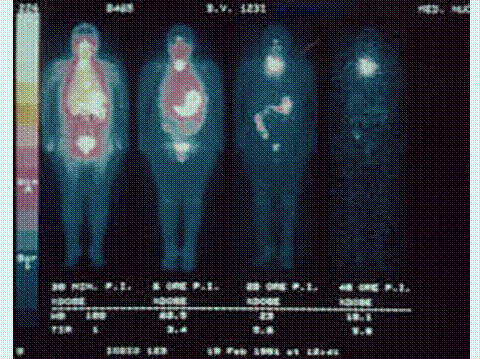
Figure 4. Sequence of I-123 total-body scintiscans of a woman after intravenous injection of I-123 (half-life: 13 hours); (from left) respectively at 30 minutes, and at 6, 20 and 48 hours. It is evident the highest and rapid concentration of radio-iodide (in white) in gastric mucosa of the stomach, salivary glands and oral mucosa. In gastric mucosa of the stomach, 131-I (half-life: 8 days) persists in scintiscans for more than 72 hours. In the thyroid I-concentration is more progressive, as in a reservoir [from 1% (after 30 minutes) to 5.8 % (after 48 hours) of the total injected dose]. Mammary gland iodide-concentration is here not evident because this woman was not pregnant or lactating. It is evident a high excretion of radioiodide in urina.
In 1996, cloning and molecular characterization of the human NIS have been performed (Dai et al. 1996; Smanik et al. 1996). In the thyroid cells active iodide transport is facilitated by three transporters: NIS, Pendrin and Apical Iodide Transporter. Expression of all three transporters appears in extrathyroidal tissues (Burbridge 2005; Rodriguez et al. 2002). Iodine is present, in different concentration, in every organ and tissue of the human body, not just the thyroid gland. So far the list of these iodide-concentrating cells includes: white blood cells, salivary and lachrymal glands, ciliary body of the eye, renal cortex, the pancreas, the liver, gastric, small and large intestinal mucosa, nasopharynx, choroid plexus, skin, adrenal cortex, mammary gland, placenta, uterus and ovary (Brown-Grant 1961). What role does iodide play in animal cells? In previous works Venturi et al. (2000a, b) reported that it is possible to hypothesize on the basis of the phylogenesis and embryogenesis three ways of action of iodine: 1) An ancient and direct action, on endodermal fore-gut, stomach and on ectodermal epidermis, where inorganic iodides probably act as antioxidants. 2) A more recent and direct antioxidant action, on salivary glands and mammary glands, thymus, ovary and on nervous, arterial and skeletal systems. 3) A more recent indirect (but also via non-genomic effects) action of the thyroid and its iodinated hormones, on all vertebrate cells, which make use of specific iodine-compounds: T4 and T3, which act in very small quantities and utilize T3-receptors. Indeed thyroid hormones contain less than 1/30 of total iodine amount. Data from Wolff (1964), Evans et al. (1966), Goethe (1999) and Venturi et al. (1985, 1999, 2000a, b, 2003) suggest that both these actions of iodine may still have an important place in the cells of modern vertebrates. Broadhurst et al.(2002) and Cunnane (2005) suggested that early Homo sapiens, living around the Rift Valley lakes and up the Nile Corridor into the Middle East, received iodine and n-3 fatty acids from littoral food resources. Dobson (1998) suggested that Neanderthal man suffered I-deficiency disorders caused by inland environment or by a genetic difference of his thyroid compared to the thyroid of the modern Homo Sapiens. I-deficient humans, like endemic cretins, suffer physical, neurological, mental, immune and reproductive diseases. Iodine has favored the evolution of the nervous system for a better adaptation to terrestrial environment as recently reported by Cunnane (2005), who suggested that “iodine is the primary brain selective nutrient in human brain evolution.” Cordain et al.(2005) recently reported that the significant changes in diet, that began with the introduction of agriculture and animal husbandry approximately 10,000 years ago, occurred too recently on an evolutionary time scale for the human genome to adjust. In conjunction with this discordance between our ancient, genetically determined biology of hunter-gatherers and fisher-gatherers human societies and the nutritional patterns of contemporary Western populations, many of the so-called diseases of civilization have emerged. Cordain suggests that iodine deficiency was probably one, among other dietary variations, introduced during the Neolithic and Industrial Periods, which have altered crucial nutritional characteristics of ancestral human diet (simultaneously fiber and polyunsaturated fatty acid contents). The evolutionary collision of our ancient genome with the nutritional qualities of recently introduced foods may underlie many of the chronic diseases of Western civilization.
Fig. 5. Daily dietary intake of iodine, according to Food and Nutrition Board, Institute of Medicine, 2001. Note that an optimal iodine intake of 6.0 mg for breast has been reported recently by Kessler in °Breast J. 2004, 10:328-36
In 1883, Kocher observed that atherosclerosis, a suspected ROS-caused disease, frequently appeared following thyroid extirpation and suggested that hypothyroidism may be causally associated with atherosclerosis. In 1930-50’s, potassium iodide was used empirically in patients with arteriosclerosis and cardiovascular diseases by European physicians (Cann 2006), and Turner (1933a, b) reported the efficacy of iodine and desiccated thyroid in preventing the development of atherosclerosis in rabbits. The antioxidant action of iodides has also been described in brain cells of rats (Katamine 1985) and in the therapy of some human chronic diseases of cardiovascular and articular systems (Winkler et al. 2000). Liu (2000a, b) showed that ROS and lipid peroxidations increase in I-deficient rats and children. Recently, Aceves et al. (2005) and Cann (2006) suggested that iodides seem to have preventive effects in breast cancer and in cardiovascular diseases. Iodine seems to support anticarcinogenic defense of the organism. Some data evidenced that iodine can prevent breast and gastric cancer in humans (Eskin 1970, 1977; Venturi 2000b, 2001; Funahashi et al.1996, 2001; Smyth 2003a, b; Kessler 2004; Szybinski et al. 2004; Abnet et al. 2006 ). Aceves et al. (2005) reported that “Iodine is a gatekeeper of mammary gland integrity” and proposes that an iodine supplement should be considered as an adjuvant in breast cancer therapy. In the same I-deficient territories human, animal and plant pathologies by I-deficiency frequently coexist. Many researchers reported that Tasco-Forage, an (iodine-rich) extract from the brown seaweed Ascophyllum nodosum, has increased antioxidant activity and immune system in both plants and in grazing animals ( Saker et al. 2001, 2004; Montgomery et al. 2001; Allen et al. 2001; Fike at al. 2001). The evolutionary adaptation of terrestrial vertebrates to environmental iodine deficiency has not finished yet (Cabello et al. 2003), and most humans and terrestrial animals still need a dietary iodine supplementation (Food and Nutrition Board, USA, 2001). According to current W.H.O. statistics more than 3 billion people in the world live nowadays in I-deficient countries. In the analysis of “National Health and Nutrition Examination Surveys” data of moderate to severe iodine deficiency is present now in a significant proportion (11.7%) of the U.S. population, with a clear increasing trend over the past 20 years, caused by reduced iodized table salt usage (Hollowell et al. 1998). In conclusion, we suggest that iodide acts as a primitive antioxidant and apoptosis-inductor with a presumed anti-tumoral and anti-atherosclerotic activity. Studies of molecular evolution of primitive antioxidants might provide the basis for further research into “new” active substances against these pathologies. |
|||||