The Bohrís model of the atom explains perfectly well the surprising regularities of the lines spectrum emitted by the atoms, but this model has some insubstantialities and defects:
Starting from the ideas of Planck and Einstein, Louis de Broglie introduced a new hypothesis: since the light behaves in some cases as a wave and in others as made up of particles, why canít particles themselves have the same dual nature too?
The pioneering idea of de Broglie was that electrons, besides being particles, could have wavelike properties too.
Planck and Einstein statement:
An electromagnetic wave (light) with frequency ![]() and wavelength
and wavelength ![]() ,
can be described as made up of particles called Photons, each Photons have Energy
,
can be described as made up of particles called Photons, each Photons have Energy ![]() and Linear Momentum
and Linear Momentum ![]() computed as follows
computed as follows
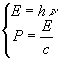 (
(![]() is
the Planckís constant and
is
the Planckís constant and ![]() is
the speed of light into vacuum.)
is
the speed of light into vacuum.)
de Broglie statement:
A particle with mass ![]() ,
while moving with speed
,
while moving with speed ![]() gets a linear Momentum
gets a linear Momentum ![]() and an energy
and an energy ![]() .
This particle can be thought as a wave irradiating in space with a frequency
.
This particle can be thought as a wave irradiating in space with a frequency ![]() and a wavelength
and a wavelength ![]() computed by reversing the previous system and coupling it with the relationship
computed by reversing the previous system and coupling it with the relationship ![]()
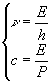 ,
, 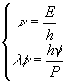 ,
,
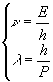 de
Broglie relationships
de
Broglie relationships![]() is
called de Broglie wavelength.
is
called de Broglie wavelength.
In this way if we know the Linear Momentum ![]() and the Energy
and the Energy ![]() of an electron, we can think it as a wavelike entity whose wavelength and
frequency are given by de Broglie relationships.
of an electron, we can think it as a wavelike entity whose wavelength and
frequency are given by de Broglie relationships.
By depicting electron in the atom like a standing wave on a circular orbit, de Broglie could easily explain Bohrís Third Postulate:
In order to form a standing wave on a circular orbit, the wavelength of the
electron must be restricted to values whose multiples ![]() equal
the circumference of the orbit:
equal
the circumference of the orbit:
If this condition is not verified the electron standing wave canít exist in the atom.
See the text figure 17.22 page 359 and try this interactive java applet:
http://www.colorado.edu/physics/2000/quantumzone/debroglie.html
By solving the system
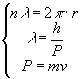
de Broglie condition explains and leads us to the third Bohrís
postulate on allowed orbits ![]() .
.
One year after the suggestion of de Broglie, Erwin SchrŲdinger started to develop a theory of the atom that used three-dimensional standing waves to describe the orbits of the electron around the nucleus.