In 1913 Niels Bohr proposed a new revolutionary model of the atom by combining the Planckís and Einsteinís researches into the light with the Rutherfordís model of the atom.
In order to overcome the difficulties connected with the Rutherfordís model he added to the classical Physics some new quantum conditions (known today as Bohrís postulates) completely in contrast with the knowledge of his time.
First Bohrís postulate states:
In the atom, electrons can turn around the nucleus only following some particular circular orbits and they do this without emitting energy. (stable orbits).
Each stable orbit has a definite energy ![]() .
.
Second Bohrís postulate states:
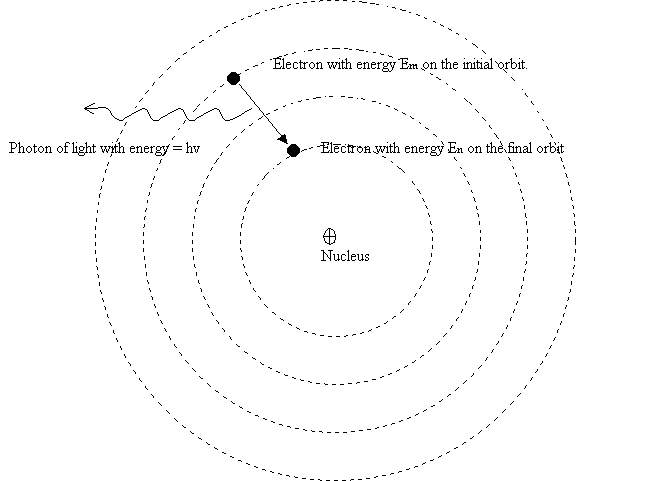
An atom emits, under the form of light, the energy ![]() only when an electron jump from an higher energy orbit to a lower energy orbit .
only when an electron jump from an higher energy orbit to a lower energy orbit .
This emitted energy is equal to the difference between the energies of the two orbits involved in the jump.
By looking at the diagram this means:
![]()
Bohr developed his predictive model for the hydrogen atom.
It is the simplest atom: it has a nucleus made of one proton and it has just one orbiting electron, hence it is quite easy to describe such a system using the physics rules.
Bohr started from the classical Newtonís equation.
![]()
is the mass of the electron.
is the Coulombís attractive force between the proton of the nucleus and the electron.
In this formula:
is the electron charge
is the proton charge.
is the Coulombís constant.
is the distance between the proton and the electron and it represents the orbit radius.
is the centripetal acceleration experienced by the electron while turning round the nucleus.
In this formula
represents the velocity of the electron.
In This way he wrote the classical motion equation for the electron in the hydrogen atom:
![]()
At this point he introduced a new Quantum limit represented by a third postulate:
Third Bohrís postulate:
The Angular Momentum of the electron ![]() in the atom, can assume only multiple values of the Planckís constant
in the atom, can assume only multiple values of the Planckís constant ![]() divided by
divided by ![]() :
:
![]()
(![]() is an integer
number that can have the values
is an integer
number that can have the values ![]() and
so on.)
and
so on.)
By writing a mathematics system between the classical motion equation (1) and the third postulate (2), Bohr discovered the radius of the possible quantum orbits for the electron in the atom:
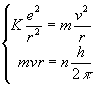
By solving this system, he found out the radius of the stable orbits of the electron.
![]()
![]()
![]()
![]()
![]()
and so onÖ.
Unfortunately the radii of the orbits canít be physically observed, only the energies emitted from the atom can. Hence, in order to have an experimental confirmation of his model, Bohr computed the energies of every single orbit too. To do this, he combined the classical considerations about the energy with the last quantum condition about the orbitís radii (3).
He found out that energies behave this way:
![]()
where ![]() is the energy of the smallest orbit
in the atom. (eV means electron-volts, a particular unit of measurement of the
energy)
is the energy of the smallest orbit
in the atom. (eV means electron-volts, a particular unit of measurement of the
energy)
The energies of the other orbits are
![]()
![]()
![]()
and so onÖ

As we can see, the bigger are the radii of the orbits, the bigger become the energies (energies become less negative). This is an expected fact: the electron needs more energy to jump to a farer orbit because it has to win the electrostatic attraction of the nucleus.
When we supply energy to the hydrogen atom, the electron can absorb it: in this case it jumps towards a more external orbit because it has a surplus of energy.
If we stop to supply energy, after a bit, the atom comes back to its normal state and jump towards the more internal orbits by emitting energies under the form of light (photons), see the java applet at http://www.colorado.edu/physics/2000/quantumzone/lines2.html.
The second Bohrís postulate said us that the energy of the emitted photon is equal to the difference between the energy of the orbits involved in the electron jump.
For example, by jumping from an higher energy orbit ![]() towards a lower energy orbit
towards a lower energy orbit ![]() ,the
electron emits one photon of light with energy
,the
electron emits one photon of light with energy
![]()
by replacing ![]() in this equation with the
(4) equation, we have
in this equation with the
(4) equation, we have
![]()
and if we write the frequency ![]() as a function of the wavelength of the photon
as a function of the wavelength of the photon ![]()
(![]() that implies
that implies ![]() )
we have
)
we have
![]()
![]()
![]()
![]()
As shown, The Bohrís model can exactly explain the energy emitted by the atom and its line spectrum.
Bohrís accomplishment was to build a model by combining different ideas available at his time:
Bohr was not seized with fright to build a model in which some new quantum conditions are superimposed to the classical motion equations. With his work, he definitively undermined the role of the Classical Physics: The whole universe canít be described by using only the Newtonís physics equation and his classical consequences.