
Emile Clapeyron (1799 - 1864)
Clapeyron was the first man to graph the transformations.
J.Watt had yet used those diagrams but he hadn't divulged them.
Clapeyron was the first man to graph the transformations.
J.Watt had yet used those diagrams but he hadn't divulged them.

In a isobaric transformation the volumes are directly proportionals to the thermodynamics temperatures.
The inlet stroke in a steam engine is an isobaric stroke.
For the thermodynamics temperatures is used the Celsius scale but the thermodynamic scale begin from -273°C (the absolute zero).

When the exhaust-valve of a steam engine opens, originates an isometric stroke.
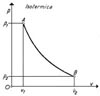
The gas temperature can to keep constant when is possible to exchange the heat with the outer ambient.
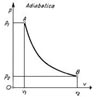
The gas temperature increases (or decreases) affecting the pressure state.