The Boyle's law and the behavior of gases.
The behavior of gases, when changes in volume and pressure occur within it, were discussed in details by Robert Boyle (1627-1691) and Edme Mariotte (1620-1684). Their results are nowadays resumed by the so-called Boyle's law. The law states that in ideal gases, if no change in temperature occurs, the pressure within the gas is inversely proportional to the volume held by it. Actually, if the volume held by the gas doubles, its internal pressure reduces to one half; if the volume becomes one third, the internal pressure increases to three times the initial one. In other words, if the volume increases the pressure decreases in inverse proportion to keep the product of the two quantities exactly constant.
![]()
This law is equivalent to the principle of conservation of energy applied to gases, and it allows us to predict how a gas changes with pressure. The diagram shows an application and refers to the sample exercise on page 165 in the book.
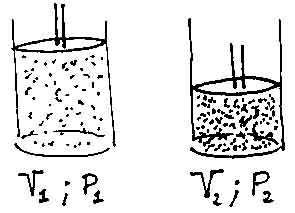
It
shows a fixed quantity of gas held in a cylinder capped at one end by
a movable piston. In the initial situation the gas holds the volume
V1 and its
internal pressure is P1. Afterwards a compression of the
piston perturbs the system putting it in a final condition of higher
pressure P2 and smaller volume V2. The Boyle's
law guarantees that the product of initial volume and initial
pressure equals the final product of the same quantities:
![]()