Our knowledge of electricity and magnetism is mainly a product of the nineteenth century. The invention of the battery by Alessandro Volta during the 1800’s allowed scientists to investigate electric phenomena systematically. As a matter of fact, the “voltaic pile” (consisting of alternating plates of copper and zinc separated by paper impregnated with a particular chemical solution) produced sustained currents for a long time and represented a device that made new experiments and investigations possible. In 1820 Christian Oersted discovered that electric current is responsible for magnetic effects too. His and the work of other scientists (due to André Marie Ampère, Michael Faraday, Joseph Henry,…) established the connection between electricity and magnetism. This connection was finally formalized some years later, when James Maxwell formulated the theory of Electromagnetism in 1865.
Nowadays we know that atoms are made of tiny different
particles which show peculiar properties.
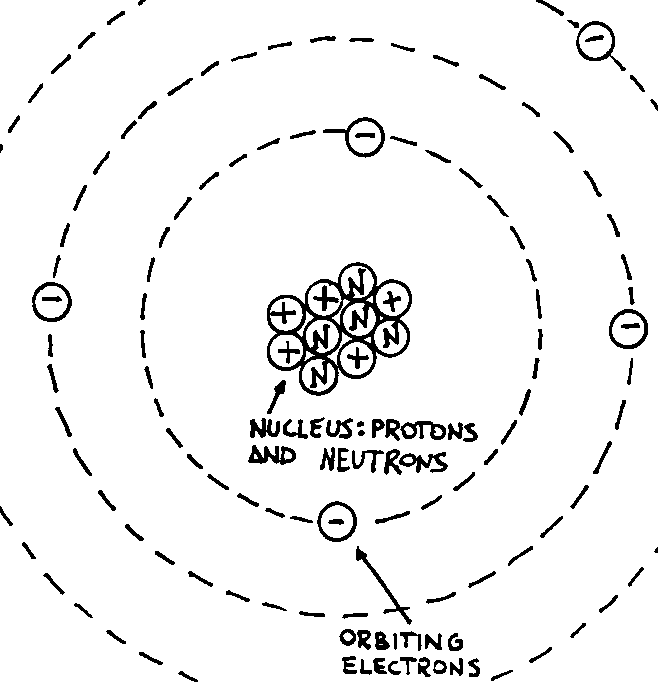
Usually the atom is depicted as a structure similar to the one in the following
figure.

Although this is an over-simplified model of an atom, it is
quite useful for our actual purposes. It consists of a central part called a
nucleus which is made of protons and neutrons. A peripheral part exists, which
consists of electrons orbiting around the nucleus.
In the usual language we say that neutrons are particles having no charge while
protons and electrons have different charges: the proton’s charge is called
“positive” and labelled with a “+” symbol; the electron’s charge is
called “negative” and labelled with a “-“ symbol. These names are a
mere, but very powerful convention, because they let us treat charges using the
algebraic tool of Maths. In any case, this description doesn’t answer the
fundamental question of what a charge is.
What is an electric charge?
The electric charge is just one of the properties that protons and electrons
have, the charge is responsible for particular kind of forces between particles,
the so-called electric forces. The charge is a property exactly like the mass
is. The mass of a particle is responsible for the gravitational attractive
force. Our experiments (pith-balls, electroscope) show that only two kinds of
charge having opposite behaviour exist. We observe that like charges
(charges of the same type) repel one another, while unlike charges
(charges of opposite type) attract one another. Hence electrical forces can be
both attractive and repulsive differing from gravitational forces that are only
attractive (this isn’t the only difference as we will see later).
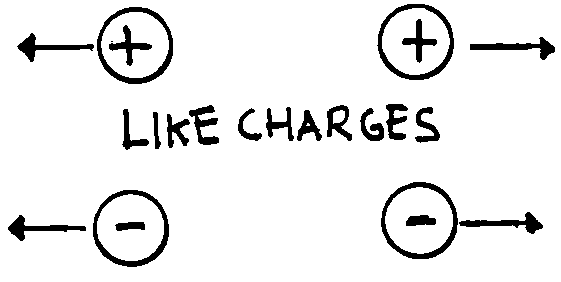
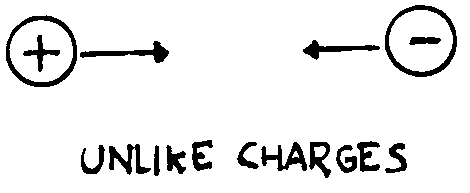
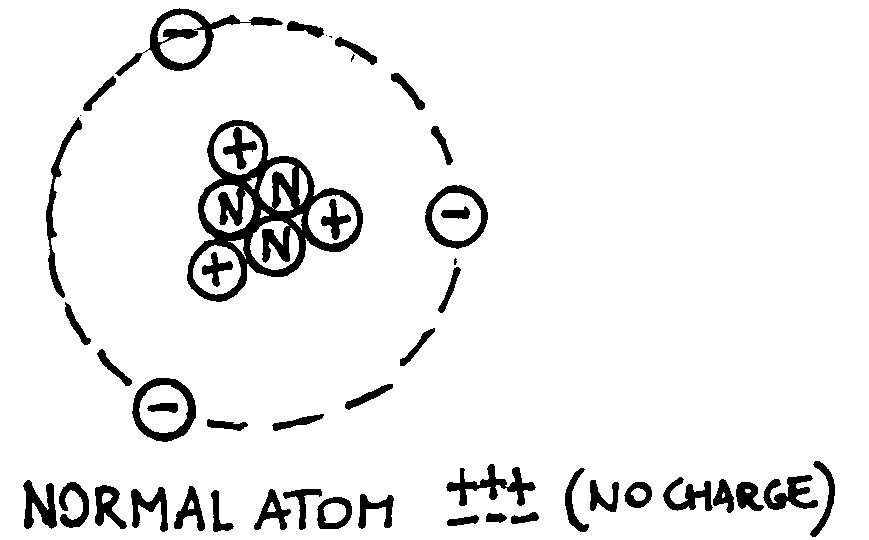
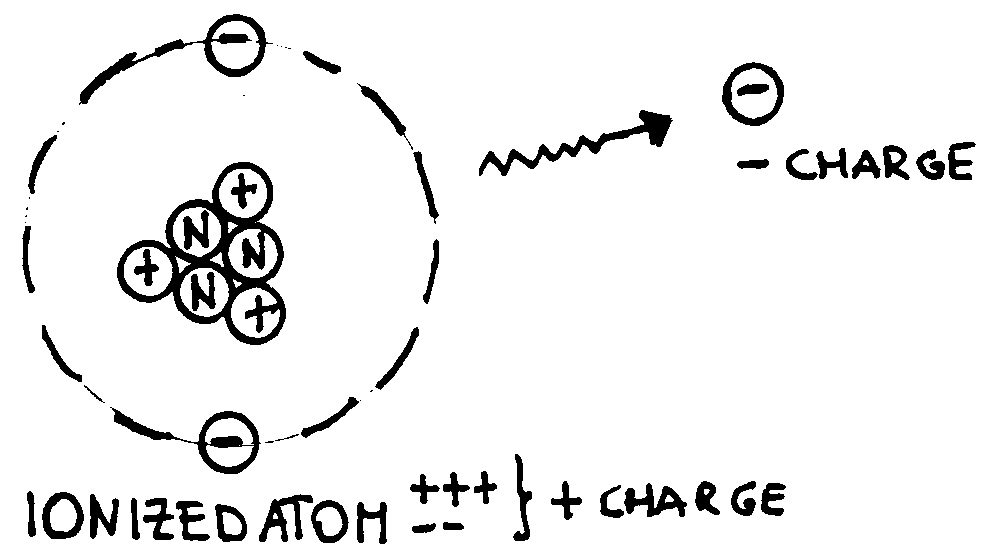
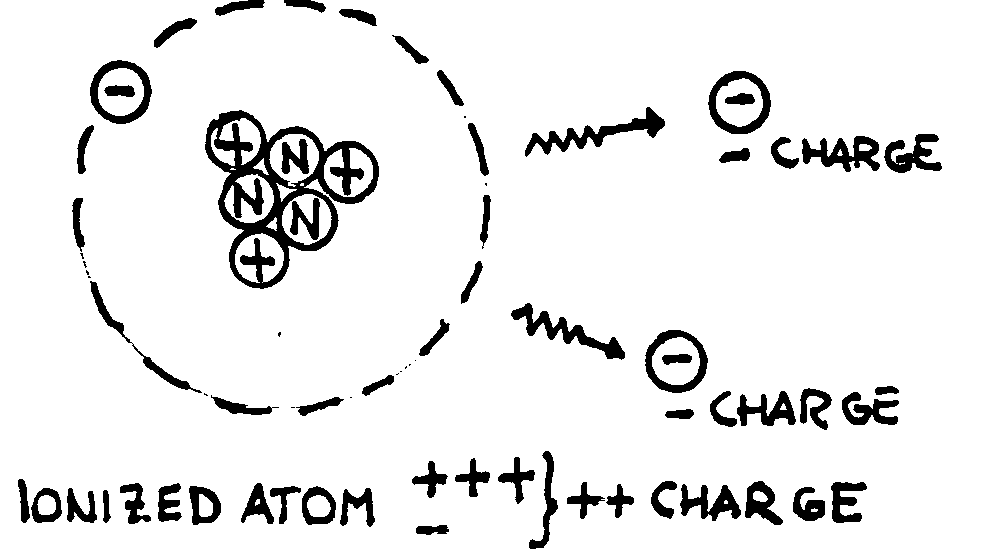
Of course, to produce electrical forces we have to produce electric charges.
Different methods exist, but each of them is based on the same idea: an atom is
neutral (has no charge) because the number of protons equals the number of
electrons. By extracting electrons from it (ionisation process) charged
particles can be created: a positive ion with a charge that is equal and
opposite to the sum of the negative charges expelled out of the atom (see the
examples below).
One way to extract electrons from atoms (or from molecules)
is by mechanical friction. For example by rubbing vigorously a plastic rod with
wool fabric, the plastic rod becomes negative and the wool positive
because the molecules of the wool lose some electrons that are captured from the
plastic molecules of the rod. Because of the mechanical friction we have a
migration of electrons from the wool to the plastic rod. The net result is a
surplus of electrons on the rod and a shortage of electrons on the wool.
Another example of electric charges production is the one obtained by rubbing a
glass rod with a piece of silk. In this case the glass rod becomes positively
charged while the silk becomes negative: some electrons are snatched to the silk
molecules from the glass molecules.
The atomic properties of materials dictate which way the electrons flow when
objects are rubbed together. Materials with atoms whose electrons are weakly
bounded to the nucleus can lose them during friction process.
Bodies can be charged also by touching and by induction, but these two methods
will be clearer after a distinguishing between insulator
and conductor materials.