Electroplatimg
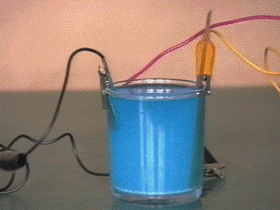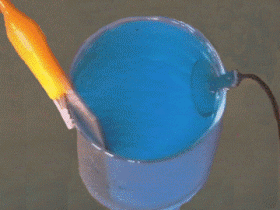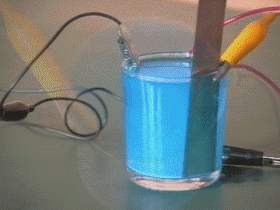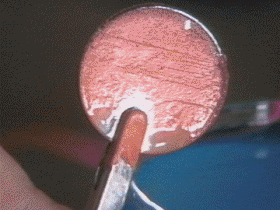
The thin coating of gold, silver, nickel or chromium that is used to adorn or protect objects is applied by the method known as electroplating.
This process is based on the principle that certain liquids are ionized when an electric current is passed trough them. The article to be plated is cleaned and suspended in a solution of the salt of the metal which is to form the coating.
The article is connected to the negative terminal of a battery and a bar of the metal to be deposited is connected to the positive terminal. As the current flows, ionized atoms of metal from the solution are deposited on the article. These atoms are replaced in the solution by others dissolved from the metal bar at the positive terminal.