Secondary batteries obtain energy by transforming certains kinds of chemicals in other kind.
When the transformation is complete the cell is dead. It can be renewed, however, by sending current from another source trough it. This restore the chemicals to their original state, and they can drive current once more.
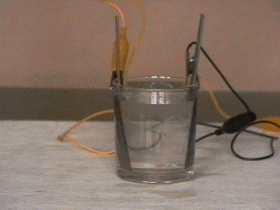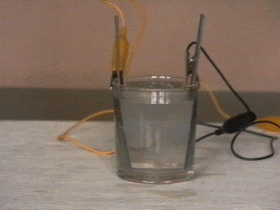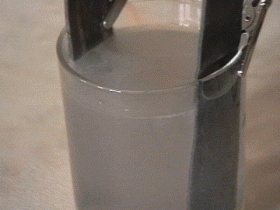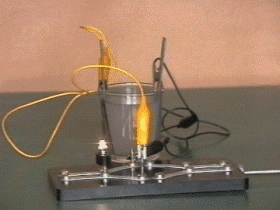
A secondary battery in its simplest form consists of two rods of lead immersed in a weak sulphuric acid solution.
Sulphuric acid (H2SO4) forms two positively charged hydrogen ions (2H+) and one negatively charged sulfate ion (-SO4).
By connecting the two rods of lead to a direct current source the ions are attracted toward them. Toward the positive rod flow the negatively charged ions (-SO4) which change the lead in red lead oxid. The negative rod of lead attracts the posive ions (H+) and becomes grey and spongy.
After a few minutes the rods must be disconnected from the direct current source, the battery is charged and now is possible to get current from it.