ELECTROCHEMICAL CELL
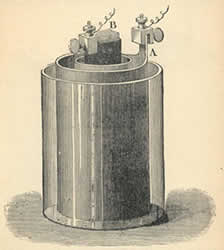 An
electrochemical cell is a device used
for creating an electromotive force
(voltage, abbreviated EMF) and current from chemical reactions. The current
is caused by the reactions releasing and accepting electrons at the different
ends of a conductor. A common example of an electrochemical cell is a
standard 1.5-volt battery. On the left, the cell invented by Robert Bunsen.
An
electrochemical cell is a device used
for creating an electromotive force
(voltage, abbreviated EMF) and current from chemical reactions. The current
is caused by the reactions releasing and accepting electrons at the different
ends of a conductor. A common example of an electrochemical cell is a
standard 1.5-volt battery. On the left, the cell invented by Robert Bunsen.
Each half-cell consists of an electrode, and an electrolyte with ions that undergo either oxidation or reduction (redox reaction). In a full electrochemical cell, ions from the electrolyte of one half-cell lose electrons (oxidation) to their electrode while ions from the electrolyte of other half-cell gain electrons (reduction) from their electrode. Finally, a salt bridge is often employed to provide electrical contact between two half-cells with very different electrolytes—to prevent the solutions from mixing. This can simply be a strip of filter paper soaked in saturated potassium nitrate (V) solution. See below for a simple schematic cell.
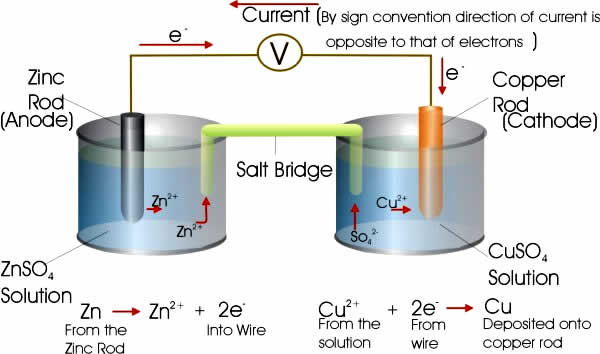
Each half-cell has a characteristic voltage. Different choices of substances for each half-cell give different potential differences. Each reaction is undergoing an equilibrium reaction between different oxidation states of the ions—when equilibrium is reached the cell cannot provide further voltage. In the half-cell which is undergoing oxidation, the closer the equilibrium lies to the ion/atom with the more positive oxidation state the more potential this reaction will provide. Similarly, in the reduction reaction, the further the equilibrium lies to the ion/atom with the more negative oxidation state the higher the potential.
This potential can be predicted quantitatively through the use of electrode potentials (the voltage measured when the substance is conventionally connected to hydrogen). The difference in voltage between electrode potentials gives a prediction for the potential measured. Click here for the standard electrode potential of the most important electrochemical elements.
If you want to know more about the different kinds of electrochemical cell, click below:
(Testo ripreso ed adattato da Wikipedia)
|
|
|

