
Near the end of the nineteenth century scientists suspected that electrical
phenomena were produced by tiny charged particles. J.J.Thomson (1856-1940)
proved this fact with an experiment on cathode rays.
Cathode rays had been observed in a glass tube, partially evacuated, with
electrodes at each end (see the figure in the textbook on page 347): if
electrodes are connected to a source of high potential difference, a glow
appears around the cathode (a cathode is the negative electrode). Thomson
demonstrated that this glow was produced by a flood of particles, all with
identical charges and mass, flowing out of the cathode. He called these
particles ELECTRONS and made the first step in determining their physical
properties by measuring their charge-mass ratio (q/m). To do this, he built a
special and completely evacuated tube like the one below.

A strong potential difference ![]() is
applied to the electrodes (cathode and anode) inserted in the left side of the
tube. Because of this strong voltage, electrons are extracted from the cathode
and accelerated towards the positive anode, they overshoot it through the hole
in its centre, and bang against the phosphor coated surface at the end of the
tube. Because of their collision, the phosphor becomes luminous and a dot-shaped
glow appears at the point where the electrons impact, revealing the direction of
the whole beam.
is
applied to the electrodes (cathode and anode) inserted in the left side of the
tube. Because of this strong voltage, electrons are extracted from the cathode
and accelerated towards the positive anode, they overshoot it through the hole
in its centre, and bang against the phosphor coated surface at the end of the
tube. Because of their collision, the phosphor becomes luminous and a dot-shaped
glow appears at the point where the electrons impact, revealing the direction of
the whole beam.
The conservation energy
principle establishes that the initial potential
energy ![]() of each electron when
at rest on the cathode, equals the total kinetic
energy
of each electron when
at rest on the cathode, equals the total kinetic
energy ![]() the electron has, when
passing through the anode. This statement can be written as
the electron has, when
passing through the anode. This statement can be written as
![]()
and leads immediately to the charge-mass ratio of the electrons:
As you can see, to determine such a ratio, besides the known voltage ![]() ,
the velocity of each electron
,
the velocity of each electron ![]() is needed. What did Thomson do to compute the velocity of the electrons in the
beam?
is needed. What did Thomson do to compute the velocity of the electrons in the
beam?
He inserted two charged plates in the middle of the tube, producing an electric
field ![]() which
was downward oriented. Since the electrons of the beam are negatively charged,
the beam deviates upward and its deviation is revealed by the displacement of
the dot-shaped glow on the phosphor coated screen (see the figure below).
which
was downward oriented. Since the electrons of the beam are negatively charged,
the beam deviates upward and its deviation is revealed by the displacement of
the dot-shaped glow on the phosphor coated screen (see the figure below).
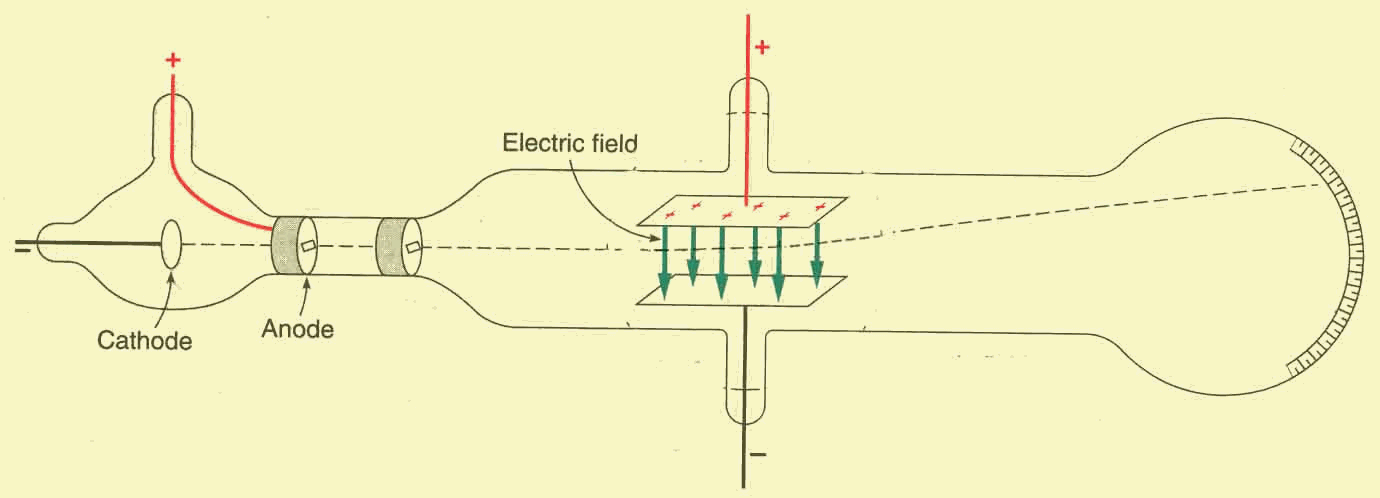
From the physical point of view, the beam deviates because an upward oriented
electric force ![]() insists on each
electron with a magnitude:
insists on each
electron with a magnitude:
![]() .
.
In order to compute the velocity of the electrons, Thompson took advantage of
the Lorentz force: the beam consists of a stream of charged particles, so it can
be deflected by a magnetic field too. Hence, in order to restore the initial
horizontal direction of the beam, he placed two external current carrying coils,
producing a magnetic field ![]() perpendicular
to the page and outward oriented from it. Such a magnetic field generates a
downward oriented magnetic Lorentz
force
perpendicular
to the page and outward oriented from it. Such a magnetic field generates a
downward oriented magnetic Lorentz
force ![]() on each electron. Its
magnitude is
on each electron. Its
magnitude is
![]() .
.
At this point, he balanced the two forces by adjusting the value of both
fields ![]() and
and ![]() (see the figure below).
(see the figure below).
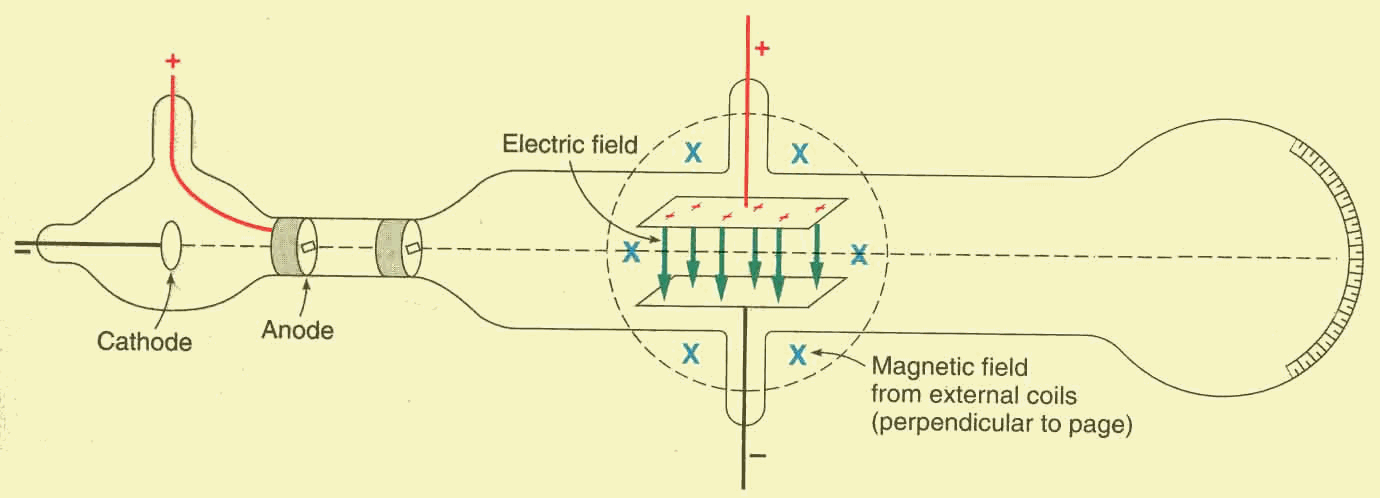
He established that the two forces were well-balanced when the glow on the phosphor screen goes back to the original position.
From the physical point of view, the balancing of the previous forces means that the electron beam flows along the original direction and that
![]() .
.
This relationship let him to compute the velocity of each electron through the ratio of the two balanced fields:
![]()
![]()
and by inserting the last one in the equation (1) Thomson finally found the value of the charge-mass ratio of the electron:
 .
.
Thomsonís experiment did not produce either the mass or the charge of the electron. It did show that the stream consists of separate and negatively charged particles, all having the same definite mass and charge. As we will see, after Thomson, Millikan used the principle of electrostatic to measure the charge on the electron; it was then a simple matter to find its mass.
Thomsonís experiment also demonstrated that atoms contained smaller and negatively charged particles: the electrons were initially thought as being uniformly distributed throughout the volume of the whole atom, like raisins in a panettone. Initially, scientists depicted the structure of the atom like the one represented in the figure above:

In this model, the atom consists of negative particles (electrons) inserted in a sphere of positive charge. Notice that there are NO protons in this primitive model.
A stream of particles is accelerated through a potential difference of 500V, and the passes straight through a pair of crossed electric and magnetic fields. If the electric field is 2600N/C and the magnetic fields is 12 mT, what is the charge to mass ratio of the particles?
First find the velocity of the particles. Since the beam passes through without deflection, the two force on the particles are equal, and

After the initial acceleration, the potential energy is completely converted to kinetic energy, so
![]()
and
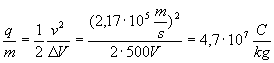 .
.
| exercise | index |